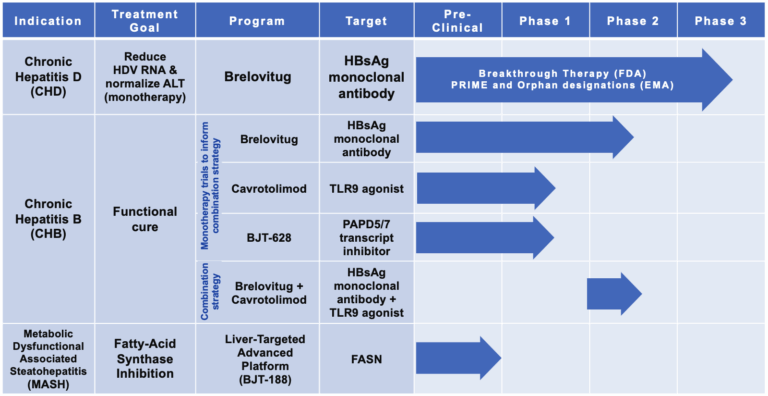
Our lead asset brelovitug (BJT-778) is a high potency, fully human immunoglobulin G1 (IgG1) monoclonal antibody (mAb) that targets the surface antigen (anti-HBsAg) of the hepatitis B virus with pan-genotypic activity. It is being investigated as a potential monotherapy for adults with chronic hepatitis D and as the foundation of a combination strategy for the functional cure of chronic hepatitis B. Brelovitug neutralizes and removes hepatitis B and hepatitis D virions and depletes HBsAg-containing subviral particles. In addition, brelovitug has shown it has the potential to be a potent immunomodulator.
Brelovitug has received Breakthrough Therapy Designation from the U.S. Food and Drug Administration, as well as Orphan and PRIME designation from the European Medicines Agency.
Our phase 3 program for brelovitug has begun with our first patient dosed in the AZURE-1 trial in March 2025. Please see more information on the trial here.