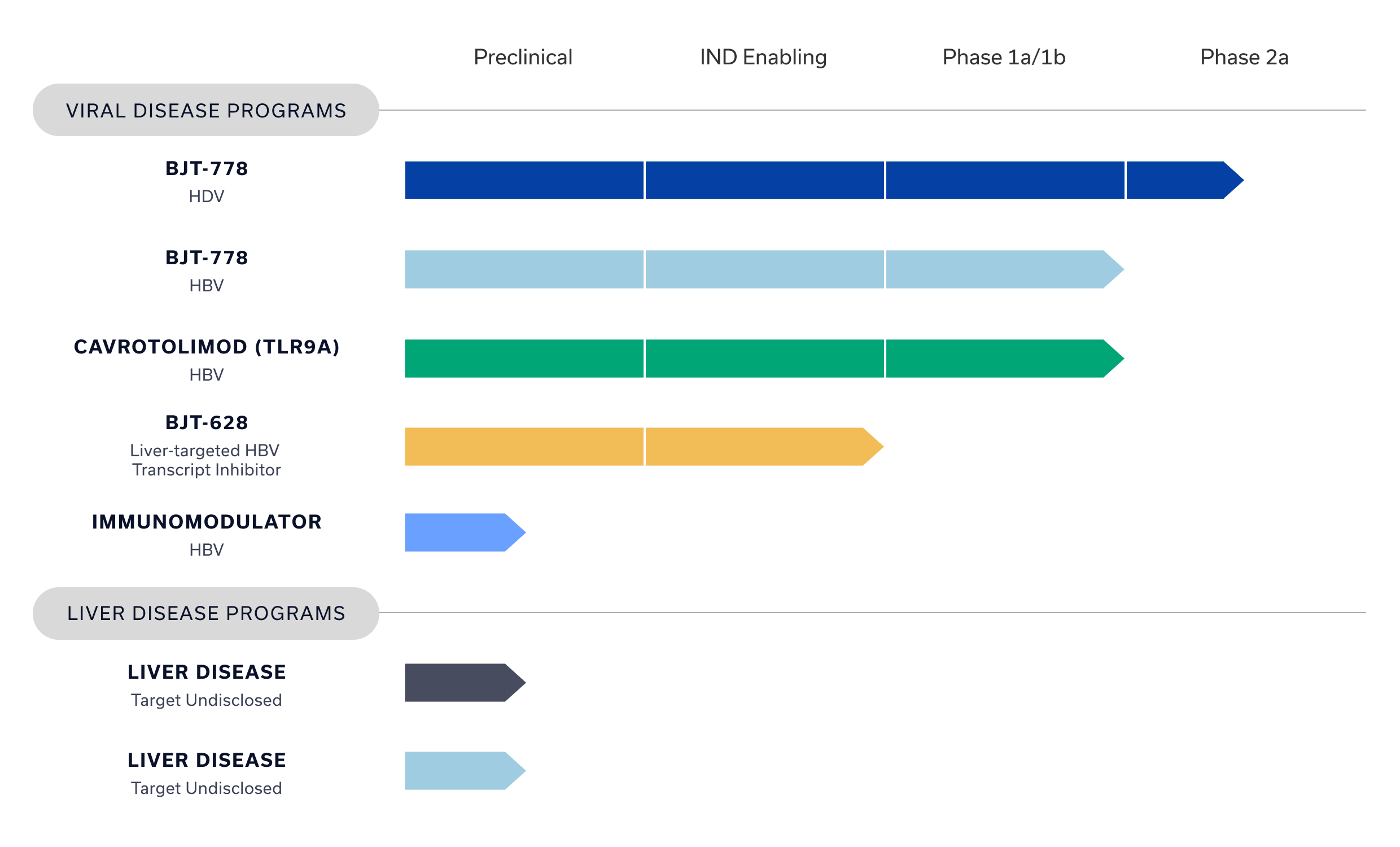
Our Pipeline
Bluejay is passionate about shifting the treatment paradigms for patients with difficult viral and liver diseases.
We’re starting by making a functional cure for CHB a reality and developing a life-changing treatment for CHD – both have been in the shadow of other infectious diseases for too long.